FES-Forward Blog
Explore the Boundaries of Synthetic Biology with us. Stay up-to-date with the latest synthetic biology and DNA synthesis news, updates, and research.
Breaking Down Barriers in DNA Synthesis: Repetitive DNA Sequences
AUG 22, 2024 │ 7 MIN READ
Explore the challenges of synthesizing and assembling repetitive DNA sequences including inverted repeats associated with Adeno-Associated Virus (AAV), short tandem repeats found in CRISPR arrays, regulatory elements and repeat expansion disorders.

Despite revolutionary advances in DNA sequencing, synthesis, and gene editing technologies, repetitive DNA sequences continue to challenge researchers due to their inherent complexity and instability. Without full fluency to read, write, and edit DNA, current molecular biology workflows have limitations, thereby imposing roadblocks to scientific discovery. In part one of this four-part series, we explore repetitive DNA sequences, where they exist naturally, and how long oligos provide new alternatives for building complex DNA constructs.
What are Repetitive DNA Sequences?
Repetitive DNA sequences are segments of the genome where specific patterns of nucleotides, the building blocks of DNA, occur multiple times in succession. These sequences can vary in size and are found in different regions of the genome, playing crucial roles in biological processes including gene expression, chromosome structure, and genome stability. These sequences can range from short tandem repeats (STR), interspersed repeats (IR), and inverted tandem repeats (ITR) (Figure 1).
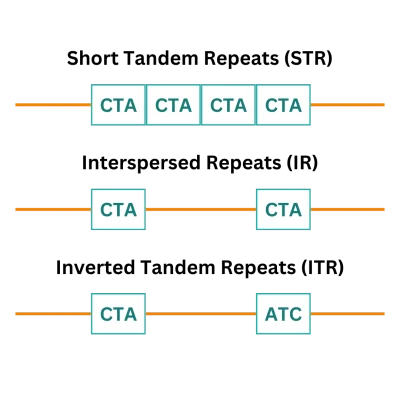
Why are Repetitive DNA Sequences Challenging in DNA Synthesis?
In DNA and gene synthesis, repetitive DNA sequences are prone to misalignment and recombination, causing non-specific binding and ultimately incorrect truncated species. Chemical synthesis has significant challenges with preparation of purine rich STR motifs, such as d(GA or AG) repeats and contiguous d(GC or CG) repeats (1,2).
Template-dependent DNA polymerase replication of repeat sequences presents its own set of challenges, including DNA polymerase slippage and stalling. Slippage occurs when the primer and template misalign during extension, leading to a range of product lengths, particularly problematic for STR motifs (3). Polymerase stalling, often triggered by inverted repeats results in truncated products or skipped regions (4,5). Addressing these issues may require the use of additives and adjusted thermal cycling conditions in PCR to enhance replication fidelity.
What are Common Examples of Repetitive DNA Sequences?
Over half of the human genome contains repetitive DNA sequences (3).
Adeno-Associated Viruses (AAVs) are commonly used in gene therapies to deliver genetic material to human cells. AAVs have ITRs that are approximately 145 nt long flanking the 5’ and 3’ end, serving as the origin of replication and being required for replication, packaging, and vector persistence (6).
The acronym CRISPR (clustered regularly interspaced short palindromic repeats) comes from a section of DNA within the bacterial genome commonly referred to as an array that has anywhere from two to hundreds of repeats that are generally ~30 base pairs long each (7).
Repeat sequences are well documented hot spots of genomic instability, with diseases like ALS (Amyotrophic Lateral Sclerosis) being characterized as an expansion of the (GGGGCC)n motif in the C9orf72 gene (8).
Microsatellites, a type of STR, are another common repetitive sequence found in non-coding regions across the genome and are often used as markers in genetic research due to their high variability. The number of repeats often varies between individuals, which makes them useful as polymorphic markers for studying inheritance patterns in families or for creating a DNA fingerprint from crime scene samples.
Repetitive DNA sequences are common in regulatory elements in eukaryotic genomes. The length and location of the repeat can influence gene expression, with many transcription factor proteins binding directly to STRs (9).
Fully Enzymatic Synthesis (FES) Enables Successful DNA Synthesis of Repetitive DNA Sequences
How can long oligos created with Fully Enzymatic Synthesis (FES) help build repetitive DNA sequences? Our FES technology was specifically engineered to overcome the challenges associated with synthesizing complex and repetitive DNA sequences, such as those found in AAV ITRs, CRISPR arrays, and disease-associated motifs like the (GGGGCC) repeat implicated in ALS.
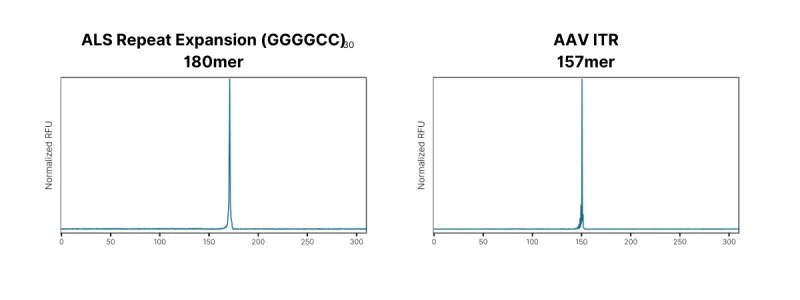
Ability to write complex sequences – Through directed evolution, our proprietary enzymatic process operates at highly elevated temperatures allowing us to melt secondary structure and build complex DNA. Figure 2 (left) shows the synthesis of the (GGGGCC)30 sequence previously mentioned, thought to be associated with ALS. Figure 2 (right) shows construction of an AAV ITR.
Long oligonucleotide building blocks – Not only is the process capable of synthesizing highly repetitive DNA sequences, having the ability to synthesize highly accurate 400mers without the need for assembly provides flexibility when designing the break points in molecular cloning and gene assembly techniques. For repetitive DNA sequences, this allows researchers to bridge repetitive DNA sequences that would cause misalignment and failure in traditional gene assembly techniques utilizing 60-100 nt oligos (Figure 3)
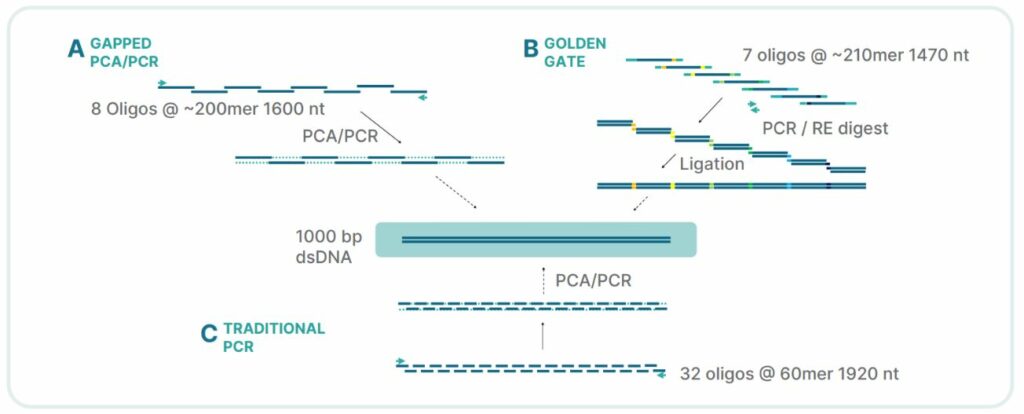
FES technology is engineered to overcome these obstacles and provide the precision and reliability needed for success. It has now been proven over a wide variety of complex sequence and molecular cloning/gene assembly techniques.
To learn more about overcoming the challenges of synthesizing high DNA complexity, download our comprehensive white paper or see the first installments of this blog series on Homopolymers and GC Content.
Explore More
Download the Complexity White Paper: Explore other complex DNA sequences by downloading our latest white paper on complexity.
Contact Us for More Information: Have questions or need more details? Reach out to our team for expert guidance on your DNA synthesis needs.
References
- Soter de Mariz E Miranda, Leandro, et al. “Editorial: Nucleosides, Nucleotides and Nucleic Acids: Chemistry and Biology.” Frontiers in Chemistry, U.S. National Library of Medicine, 10 Apr. 2024, www.ncbi.nlm.nih.gov/pmc/articles/PMC11040680/.
- Peters GJ, Sebesta, et al. “Purine and Pyrimidine Metabolism: A Firm Basis for a Transformed Society.” Nucleosides, Nucleotides & Nucleic Acids, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/17065048/. Accessed 20 Aug. 2024.
- Fan, Hao, and Jia-You Chu. “A Brief Review of Short Tandem Repeat Mutation.” OUP Academic, Oxford University Press, 15 June 2007, academic.oup.com/gpb/article/5/1/7/7210671.
- Green, Michael R. “Polymerase Chain Reaction (PCR) Amplification of GC-Rich Templates.” Cold Spring Harbor Protocols, 1 Jan. 2007, cshprotocols.cshlp.org/content/2019/2/pdb.prot095141.short.
- Saxonov, Serge. “A Genome-Wide Analysis of CPG Dinucleotides in The…” PNAS, 23 Jan. 2006, www.pnas.org/doi/10.1073/pnas.0510310103.
- Asaad, Walaa, et al. “Aav Genome Modification for Efficient Aav Production.” Heliyon, U.S. National Library of Medicine, 1 Apr. 2023, www.ncbi.nlm.nih.gov/pmc/articles/PMC10121408/#:~:text=The%20AAV%20genome%20is%20flanked,length%20%5B33%5D%20(Fig.
- Buyukyoruk, Murat, et al. “Clarifying CRISPR: Why Repeats Identified in the Human Genome Should Not Be Considered Crisprs.” The CRISPR Journal, U.S. National Library of Medicine, June 2023, www.ncbi.nlm.nih.gov/pmc/articles/PMC10277986/.
- Depienne, Christel, and Jean-Louis Mandel “30 Years of Repeat Expansion Disorders: What Have We Learned and What Are the Remaining Challenges?” American Journal of Human Genetics, U.S. National Library of Medicine, 6 May 2021, www.ncbi.nlm.nih.gov/pmc/articles/PMC8205997/.
- Connor A. Horton et al. Short tandem repeats bind transcription factors to tune eukaryotic gene expression.Science 381,eadd1250(2023).DOI:10.1126/science.add1250
